Abstract
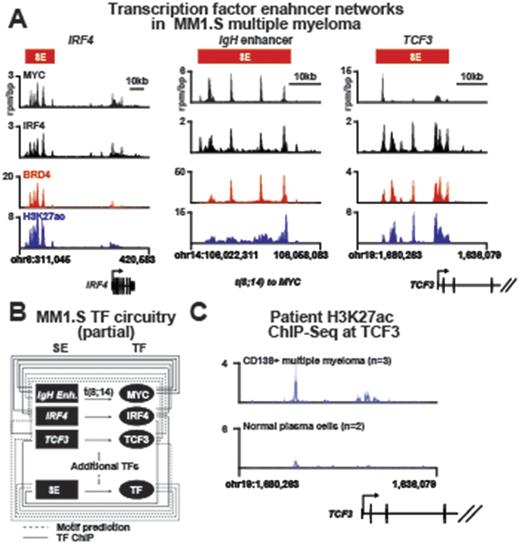
Multiple Myeloma (MM) is a complex plasma cell malignancy driven by numerous genetic and epigenetic alterations that are acquired over time. The events controlling and modifying transcriptomic changes that drive MM cell growth and progression remains undefined. To reveal the epigenetic circuitry governing myeloma cells, we performed a comprehensive analysis integrating data obtained from Multiplexed Indexed T7 Chromatin IP (Mint-ChIP), Assay for Transposase-Accessible Chromatin with high-throughput sequencing (ATAC-Seq), and RNA-seq in 10 primary MM cells as well as 3 MM cell lines to identify genome-wide the master transcription factors (TFs), the enhancer elements they occupy, and the genes they regulate. Using these data, we have identified myeloma-specific core regulatory circuitry which includes several well-established regulators of MM such as IKZF, E2F, MYC and IRF family of genes. For example, our data show elevated MYC at numerous tissue specific enhancers in myeloma cells, including those that regulate lineage specifying transcription factors such as IRF4 and TCF3 (aka E2A). When translocated to the immunoglobulin enhancer, MYC in turn is regulated by these lineage transcription factors thus integrating MYC into the interconnected transcriptional core regulatory circuitry of MM (Figure 1a,b).
We propose that this oncogenic "re-wiring" accounts for the observed addiction of MM cells to lineage factors such as IRF4 and in this work, we implicate the B-cell factor TCF3 as a novel multiple myeloma dependency. Using myeloma cell lines and primary samples, we observed elevated enhancer activity at TCF3 in primary CD138+ cells from myeloma patients compared to normal plasma cells (NPCs) (Figure 1c). As a result, TCF3 expression is significantly upregulated in our large cohort of MM patients (n=370) compared to normal bone marrow plasma cells (n=18). As MYC proteins can only bind pre-established and acetylated regions of active chromatin, we hypothesize that enhancer specifying lineage transcription factors such as TCF3 may cooperate with MYC to alter tissue specific gene expression programs. We show that TCF3 is regulated by a large proximal enhancer that is bound by MYC, and is highly sensitive to chemical perturbation of enhancer co-activators such as BRD4. As a helix-loop-helix transcription factor that similar to MYC binds short (CANNTG) E-box sequences, we computationally predict co-occupancy of MYC and TCF3 at ~80% of all enhancers that form the multiple myeloma transcriptional core regulatory circuitry. To evaluate the functional role of TCF3 in myeloma cells, we established TCF3 knock down myeloma cell lines and followed the cell growth over time. Stable knockdown of TCF3 preferentially blocks proliferation of IgH MYC translocated cell lines (such as MM1.S cells) versus non-translocated lines (such as U266 cells). Finally, high expression of TCF3 correlates with poor clinical outcome in myeloma patients. Together these data suggest TCF3 acts as an oncogenic collaborator with deregulated MYC and implicates transcriptional control of lineage as a dependency in multiple myeloma.
Figure 1: Transcriptional core regulatory circuitry of multiple myeloma: A) ChIP-Seq tracks of IRF4, MYC, BRD4, and H3K27ac occupancy at the IRF4, IgH enhancer, and TCF3 loci respectively. B) Schematic of transcription factor to enhancer connectivity of the partial multiple myeloma transcriptional core regulatory circuitry highlighting interactions between IRF4, MYC, and TCF3 (computationally predicted based on TCF3 motif data). C) ChIP-Seq tracks of H3K27ac occupancy at the TCF3 locus in patient multiple myeloma (top, n=3) or normal plasma cells (bottom, n=2).
Bradner: Acetylon: Other: Scientific Founder; Novartis: Employment. Anderson: Oncopep: Other: scientific founder; Millenium Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; C4 Therapeutics: Other: scientific founder; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees; MedImmune: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal